Answer: The number of atoms remain constant and thus the mass also remains conserved.
Step-by-step explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
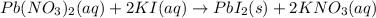
In product side ,
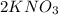 there are 2 atoms of potassium, 2 atoms of nitrogen and 6 oxygen atoms. In
there are 2 atoms of potassium, 2 atoms of nitrogen and 6 oxygen atoms. In
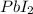 there is 1 atom of lead and 2 atoms of iodine.
there is 1 atom of lead and 2 atoms of iodine.
In reactant side ,
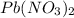 there are 2 atoms of nitrogen, 6 oxygen atoms and 1 atom of lead. In
there are 2 atoms of nitrogen, 6 oxygen atoms and 1 atom of lead. In
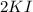 there are 2 atoms of potassium and 2 atoms of iodine.
there are 2 atoms of potassium and 2 atoms of iodine.
Thus the number of atoms remain constant and thus the mass also remains conserved.