Answer: The percent chlorine in the sample is 12.08 %.
Step-by-step explanation:
We are given:
Mass of AgCl precipitated = 0.3333 g
Molar mass of AgCl = 143.32 g/mol
Molar mass of Chlorine atom = 35.45 g
In 1 mole of AgCl, 1 mole of chlorine atom is present.
In 143.32 grams of AgCl, 35.45 g of chlorine atom is present.
So, in 0.3333 grams of AgCl, the mass of chlorine present will be =
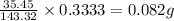
To calculate the percentage composition of chlorine in sample, we use the equation:
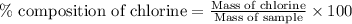
Mass of sample = 0.6789 g
Mass of chlorine = 0.082 g
Putting values in above equation, we get:
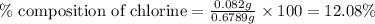
Hence, the percent chlorine in the sample is 12.08 %.