Answer:
The most spontaneous is (B)
Step-by-step explanation:
We follow the equation for Gibbs free energy:
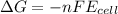
Where n is the number of electrons involved, F is the Faraday's constant (96485 C/mol). As we do not know the number of electrons per reaction, we will assume the same number for every reaction for the sake of the comparison:

With the results we can see that the reaction that would be most spontaneous is reaction b, as the Gibbs free energy is the most negative one. Also we see that reaction a occurs in the opposite direction.