Step-by-step explanation:
It is given that,
Radius of orbit,

Charge on electron,
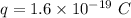
(a) The electric force exerted on each particle is given by :
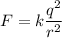
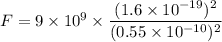
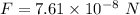
(b) If this force causes the centripetal acceleration of the electron, then we need to find the speed of the electron. Let v is the speed,
So,
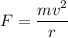
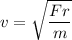
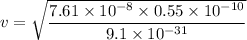
v = 2144632.96 m/s
or

Hence, this is the required solution.