Answer:
116 years
Step-by-step explanation:
For this type of problems we have to use the formula that model the radioactive decay, that it's:
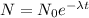 ,
,
where
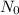 correspond to the initial amount of mass of the element,
correspond to the initial amount of mass of the element,
 correspond to the decay constant of the element,
correspond to the decay constant of the element,
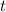 correspond the time measured in years and
correspond the time measured in years and
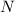 correspond to the amount of mass left after
correspond to the amount of mass left after
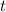 years.
years.
In this case the original amount of mass is unknown but we don't need it to resolve the question, so let's make use of the half-life data that we've been given:
If the original amount of mass is
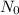 then after
then after
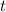 = 29 years the amount of mass left is the half of
= 29 years the amount of mass left is the half of
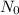 .
.
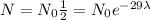 , we cancel equal terms on both sides and obtain that
, we cancel equal terms on both sides and obtain that
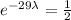 , now we take natural logarithm on both sides (due to exponential function and logarithmic function are inverses) and then we obtain
, now we take natural logarithm on both sides (due to exponential function and logarithmic function are inverses) and then we obtain
 by dividing for -29, so
by dividing for -29, so
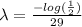
Now we know the formula that model the radioactive decay for Strontium-90:
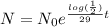
So we've been asked for the time in years were the mass left will be the 1/16 of it's original mass,
By that time
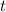 in years we have that
in years we have that
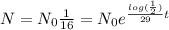 , we cancel equal terms and take natural logorithm on both sides
, we cancel equal terms and take natural logorithm on both sides
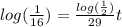 , finally we obtain after dividing that
, finally we obtain after dividing that
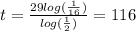 years
years