Answer:
by ideal gas pressure = 15529.475 kPa
by compressibility chart pressure = 12576 kPa
by steam tables Pressure = 12517 kPa
Step-by-step explanation:
given data
temperature T = 400°C = 673 K
volume v = 0.02 m³/kg
to find out
pressure by ideal gas, compressibility chart and steam tables
solution
we know here by table
gas constant R is 0.4615 kJ/ kg-K
and critical temp Tc = 647.1 K
and critical pressure Pc = 22064 kPa
so by ideal gas pressure is
pressure = R×T / v
pressure = 0.4615 × 673 / 0.02
pressure = 15529.475 kPa
and
by compressibility chart
temperature reduce is = T/ Tc
temperature reduce Tr = 673 / 647.1
Tr = 1.040 K
so pseudo reduce volume is here
reduce volume Vr = v / ( RTc/Pc)
reduce volume Vr =
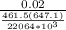
0.02 / ( 461.5(647.1) / 22064×10³)
reduce volume = 1.48
and we know by compressibility chart
reduce pressure Pr is 0.57
so
pressure = Pr × Pc
pressure = 0.57 × 22064 × 10³
pressure = 12576 kPa
and
from steam table
pressure is 12.5 MPa at 673 K and 0.020030 m³/kg
pressure is 15 MPa at 673 K and 0.015671 m³/kg
so
pressure P is
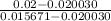 =
=
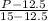
so
Pressure = 12517 kPa