Answer:
Enthalpy of reaction - 1.42 kJ/mol
Step-by-step explanation:
given data:
specific heat capacity of water = 4.18 J/g degree celcius
mass of solution = 184 g
temperature difference
 = 24.70 - 21.0 = 3.7 Degree celcius
= 24.70 - 21.0 = 3.7 Degree celcius
we know that quantity of heat is given as

q = 184*4.18*3.7
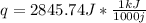
q = 2.84 kJ
Enthalpy of reaction is given as
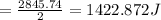
= 1.42 kJ
FOR exothermic reaction = - 1.42 kJ/mol