Answer:
The 14.59 mL of solution is needed by our lab partner.
Step-by-step explanation:
Mass of the NaCl = 1.28 g
Moles of NaCL =
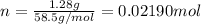
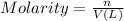
V = Volume of the solution in Liters.
 = 125.0 mL = 0.1250 L
= 125.0 mL = 0.1250 L
Molarity of our solution =

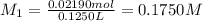
Molarity of the lab partner's solution =
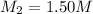
Volume of the lab partner's solution =
 = ?
= ?

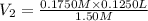
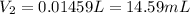
The 14.59 mL of solution is needed by our lab partner.