Answer: The ratio of the mass ratio of S to O in SO to the mass ratio of S to O in
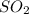 is 2.
is 2.
Step-by-step explanation:
We are given:
Mass of sulfur = 32 grams
Mass of oxygen = 16 grams
A chemical compound having a chemical formula of SO
Mass of Sulfur in SO = 32 g
Mass of Oxygen in SO = 16 g
Mass ratio of Sulfur to oxygen in SO =
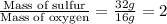 .....(1)
.....(1)
- Taking
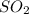 molecule:
molecule:
A chemical compound having a chemical formula of
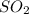
Mass of Sulfur in
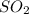 = 32 g
= 32 g
Mass of Oxygen in
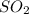 = (2 × 16) = 32 g
= (2 × 16) = 32 g
Mass ratio of Sulfur to oxygen in
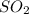 =
=
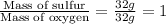 .....(2)
.....(2)
Taking ratio of 1 and 2, we get:
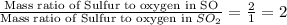
Hence, the ratio of the mass ratio of S to O in SO to the mass ratio of S to O in
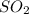 is 2.
is 2.