Answer: There will be 0.00002 meq per Liter of the solution.
Step-by-step explanation:
Normality is defined as the umber of gram equivalents dissolved per liter of the solution.
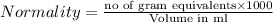
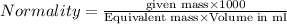
Equivalent weight is calculated by dividing the molecular weight by n factor.
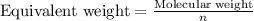
n= charge for charged species , For
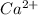 , n =2
, n =2
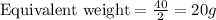
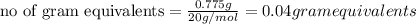
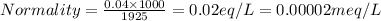
Thus there will be 0.00002 meq per Liter of the solution.