Step-by-step explanation:
As molarity is the number of moles placed in a liter of solution. Therefore, no. of mole = Molarity × volume of solution in liter
Hence, moles of
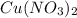 will be calculated as follows.
will be calculated as follows.
No. of mole of
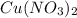 =
=
 = 0.0168 mole
= 0.0168 mole
According to the given reaction, 2 mole of
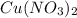 react with 4 mole of KI.
react with 4 mole of KI.
Therefore, for 0.0168 mole amount of
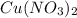 required will be as follows.
required will be as follows.
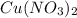 =
=
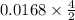
= 0.0337 mole of KI
Hence, volume of KI required will be calculated as follows.
Volume =
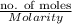
Volume of KI =
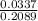
= 0.1614 liter
= 161.4 ml (as 1 L = 1000 mL)
Thus, we can conclude that 161.4 ml volume of a 0.2089 M KI is required for the given situation.