Answer: 1424 grams
Step-by-step explanation:
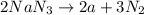
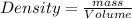
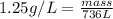
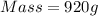
Thus mass of nitrogen produced is 920 g.

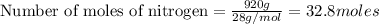
According to stoichiometry:
3 mole of
 are produced from= 2 mole of
are produced from= 2 mole of
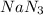
Thus 32.8 moles of
 are produced from=
are produced from=
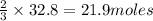 of
of
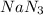
Mass of
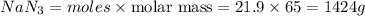
Thus 1424 grams of sodium azide is required to produce 736 L of Nitrogen gas with the density of 1.25 g/L.