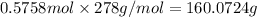Step-by-step explanation:
Mass of compounds = Moles of compound × Molecular mass of compound
a) Moles of LiCl = 2.345 mol
Molecular mass of LiCl = 42.5 g/mol
Mass of 2.345 moles of LiCl = 2.345 mol × 42.5 g/mol = 99.6625 g
b) Moles of acetylene = 0.0872 mol
Molecular mass of acetylene= 26 g/mol
Mass of 0.0872 moles acetylene= 0.0872 mol × 26 g/mol = 2.2672 g
c) Moles of sodium carbonate=
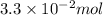
Molecular mass of sodium carbonate= 106 g/mol
Mass of
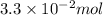 sodium carbonate
sodium carbonate
=
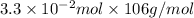 = 3.498 g
= 3.498 g
d) Moles of fructose =
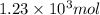
Molecular mass fructose= 180 g/mol
Mass of
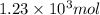 fructose
fructose
=
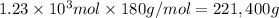
e) Moles of
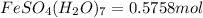
Molecular mass of
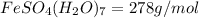
Mass of
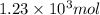 fructose
fructose
=