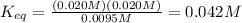Answer:
Step-by-step explanation:
Given decomposition reaction:
- 1PCl₅ (g) ⇄ 1PCl₃ + 1Cl₂(g)
Equilibrium constant:
Stoichiometric coefficients and powers equal to 1 are not usually shown as they are understood, but I included them in order to shwow you how they intervene in the equilibrium expressions: each concentration is raised to a power equal to the respective stoichiometric coefficient in the equilibrium equation.
So, your calculations are: