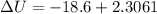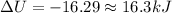Answer:-16.3 kJ
Step-by-step explanation:
Given
Pressure inside Cylinder=52,600 Pa
Piston Moved Slowly inwards 0.15 m
Area of piston(A)
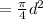
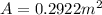
Change in volume=A\Delta L[/tex]
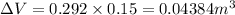
Heat removal=18,600 J
From First Law of thermodynamics
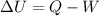
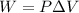
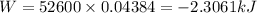
as volume is decreasing
Q=-18.6 kJ