Answer:
17,932.69 g/mol is the molecular weight of the substance.
Step-by-step explanation:
Using Beer-Lambert's law :
Formula used :
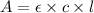
where,
A = absorbance of solution = 1.04
c = concentration of solution =?
l = length of the cell = 1 cm
 = molar absorptivity of this solution = 18,650
= molar absorptivity of this solution = 18,650
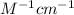
Now put all the given values in the above formula, we get the molar absorptivity of this solution.
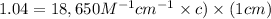
c =
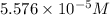
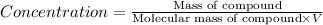
V = Volume of the solution in L
Molecular weight of the substance = x
V = 100 mL = 0.1 L
Mass of the substance = 100 mg = 0.1 g
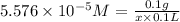
x = 17,932.69 g/mol
17,932.69 g/mol is the molecular weight of the substance.