Answer: B)
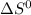 has a small negative value.
has a small negative value.
Step-by-step explanation:
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
 is positive when randomness increases and
is positive when randomness increases and
 is negative when randomness decreases.
is negative when randomness decreases.
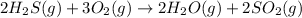
As there are 5 moles of gaseous reactants and 4 moles of gaseous products, the gaseous molecules are decreasing on moving from reactants to products, thus randomness decreases and
 is negative.
is negative.
Thus
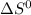 has a small negative value.
has a small negative value.