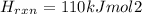Answer:
The standard enthalpy of formation of CO(g) is:
Δ
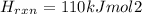
Step-by-step explanation:
The chemical equation to formation of CO is:
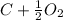 →
→
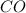
So, we need to find the standard enthalpy of formation of CO(g) with the expression:
Δ
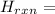 ΣΔ
ΣΔ
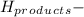 ΣΔ
ΣΔ
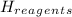 (1)
(1)
With the information of the exercise we know:
AH [C] = -393 kJ mol2
AH [CO] = -283 kJ mol2
The enthalpy of formation of a pure compound is zero so the enthalpy of Oxygen is zero.
Now, we can replace this values in the equation (1)
Δ
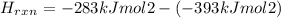
Δ