Step-by-step explanation:
The given chemical reaction will be as follows.
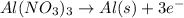
Also, mass deposited can be calculated using the formula as follows.
W = Zit
where, W = weight or mass of the substance
Z = electrochemical equivalent
i = current
t = time in seconds
Calculate value of Z for the given reaction as follows.
Z =
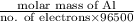
molar mass of Al = 26.98 g/mol
Z =
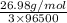
=
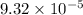 g/mol
g/mol
Therefore, putting the given values into the above formula as follows.
W = Zit
=
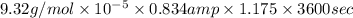
= 0.328 g
Thus, we ca conclude that 0.328 g of aluminium can be electroplated in the given situation.