Answer:
The final pH will be 7.0 (C)
Step-by-step explanation:
The pH is a scale to measure the concentration of protons in a solution. The followiing equation shows the mathematical definition of pH:
![pH = - log [H+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/zuaacttvnh4i0ifhdqmhi7vaalygllfeeb.png)
Another important equation is the one correlating the values of pH and pOH, which regards the concentration of hidroxide ions in solution;
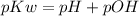 , with pKw = 14
, with pKw = 14
Finally, it is necessary to know that when an acidic and an basic solutions are mixed quantitatively, menaing with no excess of hydrogen or hydroxide ions, a neutralization reaction occurs:
HCl + NaOH → NaCl + H₂O
Now, lets calculate the H⁺ and OH⁻ concentrations in both solutions:
HCl: pH = 1.0, which means pH = -log[H⁺] ∴ [H⁺] = 1 x 10⁻¹ mol/L
NaOH: pH = 13.0, which means pOH = 1.0 pOH = -log[OH⁻] ∴ [OH⁻] = 1 x 10⁻¹ mol/L
Since the concentrations of H⁺ and OH⁻ are the same and equal volumes of each solutions were used, a neutralization reaction will happen and the final pH will be equal to the pH of water (pKw) which is 7.0.