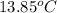Answer : The water's temperature change will be,
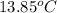
Explanation : Given,
Density of water = 0.998 g/mL
Volume of water =
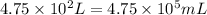
(conversion used : 1 L = 1000 mL)
Specific heat of water =
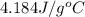
Heat absorbed =
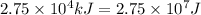
(conversion used : 1 kJ = 1000 J)
First we have to determine the mass of water.
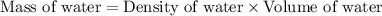
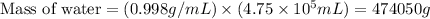
Now we have to calculate the change in temperature of water.
Formula used :
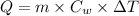
where,
Q = heat absorbed by water
m = mass of water
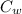 = specific heat of water
= specific heat of water
 = change in temperature
= change in temperature
Now put all the given value in the above formula, we get:
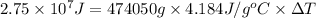
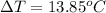
Therefore, the water's temperature change will be,