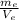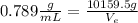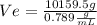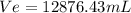Answer:
Ve=12876.43mL : Ethanol volume
Step-by-step explanation:
We apply the formula to calculate the density:
ρ=
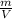 Formula (1)
Formula (1)
Where:
ρ:Density in g/mL
m: mass in g (grams)
V= Volume in ml (milliliters)
We know the following data:
1L= 1000mL
Vm=Volume of mercury:VHg=0.750 L*1000mL/L=750mL
ρ-m=Density of mercury =13.546 g/mL
ρ-e: density of ethanol is 0.789 g/mL
Development of the problem:
In formula 1 We replace the known data for the mercury :
ρ-m=
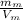
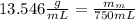
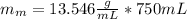
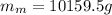
We know that
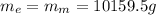 , then, We replace the known data for the ethanol In formula 1:
, then, We replace the known data for the ethanol In formula 1:
ρ-e=