Answer:
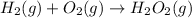
Step-by-step explanation:
Hello!
In this case, since the formation reaction of a compound is undergone when the pure elements composing it are combined, for gaseous hydrogen peroxide, gaseous diatomic hydrogen and oxygen (standard state) must be combined in order to obtain the gaseous hydrogen peroxide as shown below:
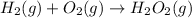
Whereas it is proved there are two hydrogen and oxygen atoms at each side of the chemical equation and therefore it is balanced.
Best regards!