Answer:
Step-by-step explanation:
Heat lost by hot copper = heat gained by water= mass x specific heat x change in temperature.
Heat lost by piece of copper
= 32 x 0.385 x ( 77.9 - 28.9)
=603.68 J
If m be the mass of water taken
Heat gained by water
=m x 4.184 x (28.9 - 27.4 )
= 6.276 m
Heat lost = heat gained
6.276 m = 603.68
m =
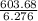
= 96.188 g
Volume of water = mass / density
=
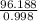
= 96.38 mL