Answer: 1.06 kg
Step-by-step explanation:
Density is defined as the mass contained per unit volume.
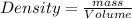
Given :
Density of mercury =
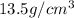
volume of cylinder = volume of mercury =
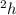
where r = radius of cylinder =
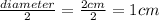
h= height = 25.0 cm
Putting in the values we get:
volume of mercury =
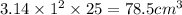
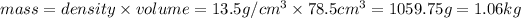 (1kg=1000g)
(1kg=1000g)
Thus mass of mercury required to fill a hollow cylinder having an inner diameter of 2.00 cm to a height of 25.0 cm is 1.06 kg.