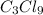Answer:
Step-by-step explanation:
Molecular Formula is representation of the chemical compound in terms of the symbols of all the elements that are present in the compound followed by subscripts, which give the count of each element in that compound.
We need to write the molecular formula of Tricarbon nonachloride. Tri means three, so Tricarbon means there are 3 atoms of Carbon. Likewise, nona stands for 9, so nonachloride means there are 9 atoms of chlorine. Therefore, we can represent nonachloride as:
Carbon (3 atoms) Chlorine (9 atoms) =
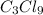
Thus, molecular formula of Tricarbon nonachloride is