Answer:
The vapor pressure is 170.6 mmHg.
Step-by-step explanation:
Given that,
Heat of vaporization = 16.69 kJ/mole
Temperature = 254.3
Pressure = 92.44 mmHg
Temperature = 275.7 K
We need to calculate the vapor pressure
Using relation pressure and temperature
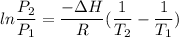
Put the value into the relation
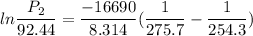
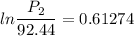
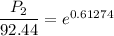
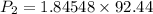
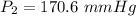
Hence, The vapor pressure is 170.6 mmHg.