Answer: Option (e) is the correct answer.
Explanation:
A compound that will dissociate in water or an aqueous solution to give hydrogen ions(
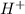 ) is known as an acidic substance.
) is known as an acidic substance.
When RbH is dissolved in aqueous solution then it dissociates as follows.
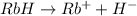 .
.
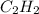 is non-polar in nature. So, it will not dissociate into ions when dissolved in an aqueous solution.
is non-polar in nature. So, it will not dissociate into ions when dissolved in an aqueous solution.
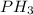 will also remain neutral and does not dissociate into ions.
will also remain neutral and does not dissociate into ions.
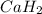 when dissolved in water then it dissociates as follows.
when dissolved in water then it dissociates as follows.
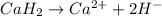
Whereas when HF is dissolved in aqueous solution then the reaction will be as follows.
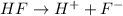
Thus, we can conclude that out of the given options HF is the compound which will produce an acidic aqueous solution.