Answer:
The mass of steam produced would be 21.95 g
Step-by-step explanation:
The heat that the iron block would lose will be gained by water, this is expressed thus;
Heat loss by Iron = Heat gain by water;
Given that;
mass of block = 400 g / 1000 = 0.4 kg
Initial temp of iron = 400°C
mass of water = 60 g / 1000 = 0.06 kg
heat vaporization of water L (constant) = 22.6 x J/Kg
average specific heat of the iron over this temperature range is = 560 J/Kg K.
Specific heat capacity of water = 4186 J / kg K
heat lost by iron = heat gain by water .......................1
ΔT is the change in temperature.
m is the mass of steam during vaporization
Heat loss by iron = x x ΔT
= 0.4 x 560 x (400-100)
= 67200 J
Heat gained by water =
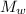
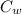 (ΔT ) + m L
(ΔT ) + m L
expression 1 would be;
67200 = 17581.2 + m x (22.6 x )
Making m the subject formula we have;
m = ( 67200-17581.2 ) / ( 22.6 x )
m = 0.0219 kg x 1000 = 21.95 g
Therefore the amount of steam produced would be 21.95 g