Answer:
10.04 g of NaOH is required for neutralization
Step-by-step explanation:
neutralization reaction:

Molar mass of NaOH = 40 g/mol
Molar mass of HCl = 36.5 g/mol
So, 9.16 g of HCl =
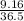 moles of HCl = 0.251 moles of HCl
moles of HCl = 0.251 moles of HCl
According to neutralization reaction, 1 mol of NaOH is required to neutralize 1 mol of HCl
So, 0.251 moles of NaOH are required to neutralize 0.251 moles of HCl
So mass of NaOH required for neutralization = (
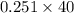 ) g = 10.04 g
) g = 10.04 g