Step-by-step explanation:
Sulfuric acid (
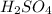 ) is a diprotic acid. So, it means that we need twice as much NaOH.
) is a diprotic acid. So, it means that we need twice as much NaOH.
As we known that an equivalence point is reached when moles of an acid equals the moles of a base.
As moles of
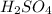 is calculated as follows.
is calculated as follows.
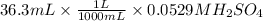 = 0.00192 moles of
= 0.00192 moles of
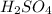
Therefore, moles of NaOH needed will be as follows.
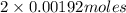
= 0.00384 mol
Hence, volume of NaOH is calculated as follows.
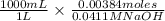
= 93.43 mL
Thus, we can conclude that the volume of base required to reach the equivalence point is 93.43 mL.