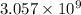Answer :
The number of gold atoms in nanogram is,
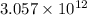
The number of gold atoms in picogram is,
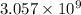
Explanation :
As we know that the molar mass of gold is, 196.97 g/mole. That means, 1 mole of gold has 196.97 grams of mass of gold.
As we know that,
1 mole contains
 number of atoms.
number of atoms.
First we have to determine the number of gold atoms in a nanogram.
As, 196.97 grams of gold contains
 number of gold atoms
number of gold atoms
And, 1 grams of gold contains
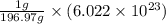 number of gold atoms
number of gold atoms
So,
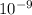 nanograms of gold contains
nanograms of gold contains
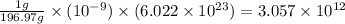 number of gold atoms
number of gold atoms
The number of gold atoms in nanogram is,
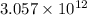
Now we have to determine the number of gold atoms in a picogram.
As, 196.97 grams of gold contains
 number of gold atoms
number of gold atoms
And, 1 grams of gold contains
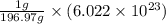 number of gold atoms
number of gold atoms
So,
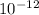 picograms of gold contains
picograms of gold contains
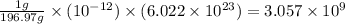 number of gold atoms
number of gold atoms
The number of gold atoms in picogram is,