Answer:
a) Qh= 6750 kJ
b) e = 0.296
c)
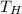 = 390.625° C
= 390.625° C
Step-by-step explanation:
Given:
Work done, W = 2000 kJ
Heat flow, Q = 4750 kJ
Temperature at which heat flows out,
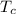 = 275° C
= 275° C
a) Now, the heat flow through the engine (Qh)
Qh = W + Q
or
Qh = 2000 + 4750
or
Qh= 6750 kJ
b) The efficiency (e) is given as:
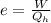
on substituting the values, we get
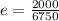
or
e = 0.296
c)
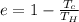
where,
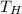 is the temperature at which heat flow
is the temperature at which heat flow
on substituting the values, we get
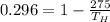
or
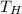 = 390.625° C
= 390.625° C