Step-by-step explanation:
The given data is as follows.
Concentration = 0.1
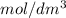
= 0.1 \frac{mol dm^{3}}{dm^{3}} \frac{10^{3}}{dm^{3}} \times \frac{6.022 \times 10^{23}}{1 mol} ions
=
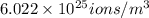
T =
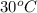 = (30 + 273) K = 303 K
= (30 + 273) K = 303 K
Formula for electric double layer thickness (
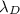 ) is as follows.
) is as follows.
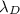 =
=
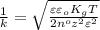
where,
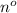 = concentration =
= concentration =
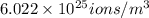
Hence, putting the given values into the above equation as follows.
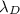 =
=
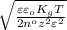
=
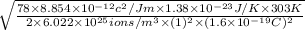
=
 m
m
or, =
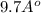
= 1 nm (approx)
Also, it is known that
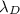 =
=
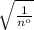
Hence, we can conclude that addition of 0.1
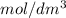 of KCl in 0.1
of KCl in 0.1
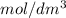 of NaBr "
of NaBr "
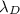 " will decrease but not significantly.
" will decrease but not significantly.