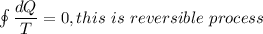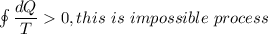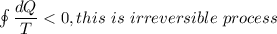Step-by-step explanation:
Second law tell us that ,the entropy of reversible process is remain constant while the entropy of ir-reversible process increases.
Entropy of universe is the summation of entropy of system and entropy of surrounding.So we can say that
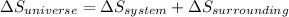
Entropy can be define as
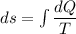
Entropy is point function.This is property of system.Ir-reversibility cause the increase of entropy.