Answer:
The amount of entropy produced by the quenching process is 0.303 kJ/K.
Step-by-step explanation:
Given that,
Mass = 2.5 kg
Temperature = 800°C
The mass of 50 L of water is 50 kg
Temperature = 20°C
Energy = 4.18 kJ/kg.K
If the final temperature of the metal bar and water is T₀°C, then
The heat lost by the bar = heat gained by water
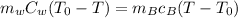
Put the value into the formula
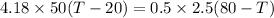
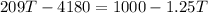
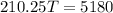
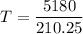
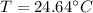
Now, heat exchange between the systems
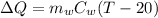
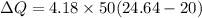
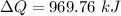
We need to calculate the entropy
Using formula of entropy
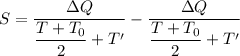
Where, T' = 273.15\ K
Put the value into the formula
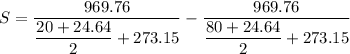
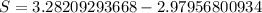
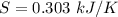
Hence, The amount of entropy produced by the quenching process is 0.303 kJ/K.