Answer: The correct answer is Option B.
Step-by-step explanation:
Percent solution by mass (% m/m) means that amount of solute present in 100 grams of solution.
Percent solution by volume (% m/v) means that amount of solute present in 100 mL of solution.
Molarity is defined as the number of moles of solute present in 1 L of solution or 1000 mL of solution.
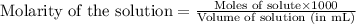
Hence, the correct answer is Option B.