Answer:
The wavelength of the visible line in the hydrogen spectrum is 434 nm.
Step-by-step explanation:
It is given that, the wavelength of the visible line in the hydrogen spectrum that corresponds to n₂ = 5 in the Balmer equation.
For Balmer series, the wave number is given by :
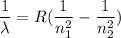
R is the Rydberg's constant
For Balmer series, n₁ = 2. So,
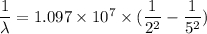
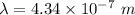
or
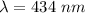
So, the wavelength of the visible line in the hydrogen spectrum is 434 nm. Hence, this is the required solution.