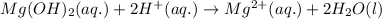Answer:
Balanced molecular equation and net ionic equation have been shown below.
Step-by-step explanation:
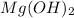 is a weak base as it does not dissociate completely in water. HCl is a strong acid as it dissociates completely in water.
is a weak base as it does not dissociate completely in water. HCl is a strong acid as it dissociates completely in water.
Reaction between
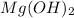 and HCl is an example of acid-base reaction. Here a salt
and HCl is an example of acid-base reaction. Here a salt
 is formed along with
is formed along with
 .
.
Balanced molecular equation:
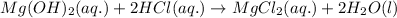
To write net ionic equation, spectator ions are to be removed from both side of total ionic equation. Here
 is the spectator ion.
is the spectator ion.
Total ionic equation:
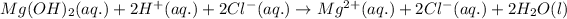
Net ionic equation: