Answer:
52.55% was the percent yield of their process.
Step-by-step explanation:
Amount of NO expected by pharmaceutical company = 7200 grams
Amount of NO actually obtained by pharmaceutical company = 3784 grams
To calculate the percentage yield, we use the equation:
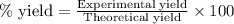
Experimental yield of nitrous oxide = 3784 grams
Theoretical yield of nitrous oxide = 7200 grams
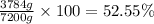
52.55% was the percent yield of their process.