Answer:
The initial state is 6 and the final state is 2.
Step-by-step explanation:
Given, wavelength of photon,
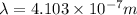
and the value of Plank's constant,
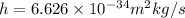
and the velocity of light,
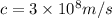
And,
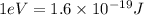
According to the Bohr model the nth level of electron will be,
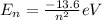
In the emission the energy is ejected out so,
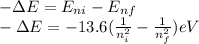
And the change in energy also,
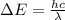
Equate above two values.
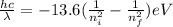
Therefore,
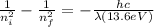
Put all the variables in the above equation
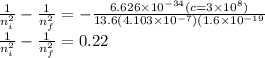
Now by putting the value of ni=6 and nf=2 to check the equality of equation.
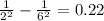
By looking at the above the value of ni=6 and nf=2 will satisfy the equation.