Answer:
96.32 %
Step-by-step explanation:
Given that:
The solubility of compound in hot water = 4.35 g / 100 mL
The solubility of compound in cold water = 0.16 g / 100 mL
Which means that in 100 mL of hot water, the dissolved compound is 4.35 g and in cold water, the dissolved compound is 0.16 g
Hence, on transition, compound that will catalyze is 4.35 - 0.16 g = 4.19 g
So,
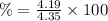
Percent recovery for re-crystallization of this compound from water= 96.32 %