Step-by-step explanation:
As it is given that stream A is super heated. From stream tables, we get that specific enthalpy, (
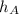 ) is 3054.29 kJ/kg.
) is 3054.29 kJ/kg.
For stream B, it is saturated water at 25 degree celsius. It's
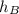 is 2546.54 kJ/kg.
is 2546.54 kJ/kg.
Stream C, data will be as follows.
P = 200 kPa,
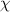 = 0.9
= 0.9
So,
 =
=

= 504.47 + 0.9 \times 2201.7
= 2486 kJ/kg
Now, energy balance formula will be as follows.
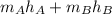 =
=
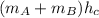
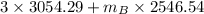 =
=
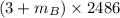
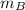 = 28.16 kg/s
= 28.16 kg/s
Hence, inflow of saturated liquid is 28.16 kg/s. According to steam table, temperature of wet steam is
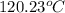
Mass flow rate of out flow is 31.16 kg/s.