Answer: The amount of heat needed is 29000 Cal.
Step-by-step explanation:
The process involved in this problem are:
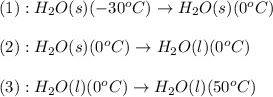
Now, we calculate the amount of heat released or absorbed in all the processes.
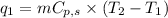
where,
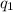 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of ice = 200 g
 = final temperature =
= final temperature =
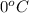
 = initial temperature =
= initial temperature =
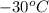
Putting all the values in above equation, we get:
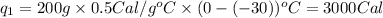
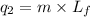
where,
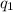 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of water or ice = 200 g
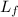 = latent heat of fusion = 80 Cal/g
= latent heat of fusion = 80 Cal/g
Putting all the values in above equation, we get:
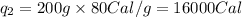
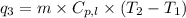
where,
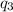 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of water = 200 g
 = final temperature =
= final temperature =
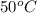
 = initial temperature =
= initial temperature =
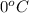
Putting all the values in above equation, we get:
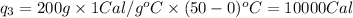
Calculating the total heat absorbed, we get:
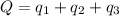
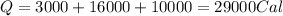
Hence, the amount of heat needed is 29000 Cal.