Answer:
a) 0.39795 kJ/K
b) 79.589.37 kJ
Step-by-step explanation:
m = Mass of air = 2 kg
Temperature = 200 K
P₁ = Initial pressure = 300 kPa
P₂ = Final pressure = 600 kPa
R = mass-specific gas constant for air = 287.058 J/kgK
a) For isentropic process
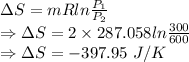
∴ Entropy is generated in the process is 0.39795 kJ/K
b)
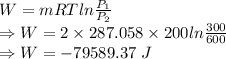
∴ Amount of lost work is 79.589.37 kJ