Answer:
h=27.15 KJ/kg
Step-by-step explanation:
We know that ,Enthalpy of perfect gas depends only on temperature it did not depends on the volume and pressure.But on the other hand enthalpy of real gas depends on the temperature and pressure.
So specific enthalpy(h) is given as follows
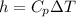
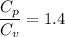
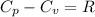
For oxygen
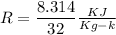
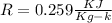
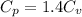
From above
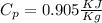
So
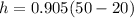
h=27.15 KJ/kg