Answer: The ratio of carbon in both the compounds is 1 : 2
Step-by-step explanation:
Law of multiple proportions states that when two elements combine to form two or more compounds in more than one proportion. The mass of one element that combine with a given mass of the other element are present in the ratios of small whole number. For Example:
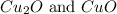
Total mass of sample = 100 g
Mass of carbon = 27.2 g
Mass of oxygen = (100 - 27.7) = 72.8 g
To formulate the formula of the compound, we need to follow some steps:
- Step 1: Converting the given masses into moles.
Moles of Carbon =
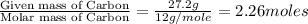
Moles of Oxygen =
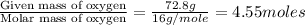
- Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 2.26 moles.
For Carbon =
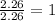
For Oxygen =
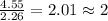
- Step 3: Taking the mole ratio as their subscripts.
The ratio of C : O = 1 : 2
Hence, the formula for sample 1 is

Total mass of sample = 100 g
Mass of carbon = 42.9 g
Mass of oxygen = (100 - 42.9) = 57.1 g
To formulate the formula of the compound, we need to follow some steps:
- Step 1: Converting the given masses into moles.
Moles of Carbon =
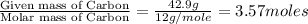
Moles of Oxygen =
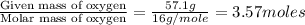
- Step 2: Calculating the mole ratio of the given elements.
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 3.57 moles.
For Carbon =
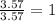
For Oxygen =
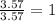
Step 3: Taking the mole ratio as their subscripts.
The ratio of C : O = 1 : 1
Hence, the formula for sample 1 is
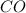
In the given samples, we need to fix the ratio of oxygen atoms.
So, in sample one, the atom ratio of oxygen and carbon is 2 : 1.
Thus, for 1 atom of oxygen, the atoms of carbon required will be =
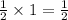
Now, taking the ratio of carbon atoms in both the samples, we get:
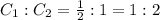
Hence, the ratio of carbon in both the compounds is 1 : 2