Answer:
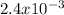
Step-by-step explanation:
We can solve this equation in a lot of different ways, the easiest equation is called ruled of three, and it's solved as follows:
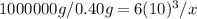 this is an equality of the information that has been given to us, and we solve it algebraically.
this is an equality of the information that has been given to us, and we solve it algebraically.
X = How much lead would be present in 6.0×103 g of blood
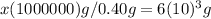
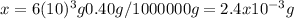
And that's how we conclude the lead content in 6*10^3g blood