Answer: The mole fraction of chloroform in this solution is 0.41
Step-by-step explanation:
To calculate the moles, we use the equation:
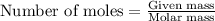
a) moles of
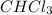
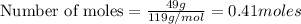
b) moles of
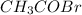
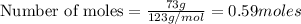
To calculate the mole fraction, we use the formula:
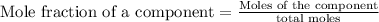
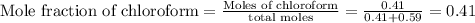
The mole fraction of chloroform in this solution is 0.41