Answer : The pH of the solution is, 10.4
Explanation :
pH : It is defined as the negative logarithm of hydrogen ion concentration.
Mathematically,
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
Given:
![[H^+]=3.5* 10^(-11)M](https://img.qammunity.org/2020/formulas/chemistry/middle-school/z8lon0ui1kp4h853lz4djf0i4qa05nu74r.png)
Now put all the given values in the above expression, we get the value of pH.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
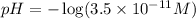
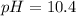
Thus, the pH of the solution is, 10.4